Quartz Cuvette: Complete & Essential Guide for Researchers (2025)
🔬 Introduction
In spectroscopy, most attention goes to the big machines—spectrophotometers, fluorometers, or the software that runs them. But one small part often decides if your data is clear or flawed: the cuvette.
A cuvette looks simple, just a small container. Yet it is the path light must travel before reaching the sensor. Even tiny flaws in the material, shape, or cleanliness can distort results.

Source: terpconnect.umd.edu
💡 For example, measuring DNA at 260 nm in a plastic cuvette will fail. Plastic blocks UV light below 300 nm, so your signal becomes weak or disappears. Using glass for fluorescence assays is also risky. Glass adds background glow, hiding weak signals and lowering accuracy.
This is why quartz cuvettes matter. They are made from fused silica and provide:
- ✅ Transparency down to 190 nm (deep UV)
- ✅ Very low background glow for fluorescence
- ✅ Strong resistance to most solvents
- ✅ Heat stability up to 150–1200 °C
Because of these strengths, quartz cuvettes are essential in:
- 🧬 Molecular biology (DNA, RNA, proteins)
- 💊 Pharmaceutical quality checks
- 🌍 Environmental and water testing
- ⚙️ Industrial research and process monitoring
👉 In a hurry? Skip ahead and [Request a Quote for Quartz Cuvettes]
1. What is a Quartz Cuvette?
A quartz cuvette is a small, precisely made optical cell that holds liquid samples for spectroscopy. It is usually rectangular and has flat, polished windows. These windows let light pass through the sample with minimal distortion. While glass or plastic cuvettes are common, quartz cuvettes are the standard whenever UV transparency, chemical resistance, and low background noise are required.
Material: Fused Silica Quartz
Quartz cuvettes are made from fused silica, a high-purity synthetic form of silicon dioxide. Natural quartz may contain impurities that absorb or scatter UV light. Fused silica avoids this problem by being produced under controlled, clean conditions, giving it exceptional optical clarity.
Key advantages of fused silica:
- Transmits light down to ~190 nm, making DNA and RNA quantification at 260 nm possible
- High transparency across visible and near-infrared ranges
- Stable, reproducible performance for sensitive assays
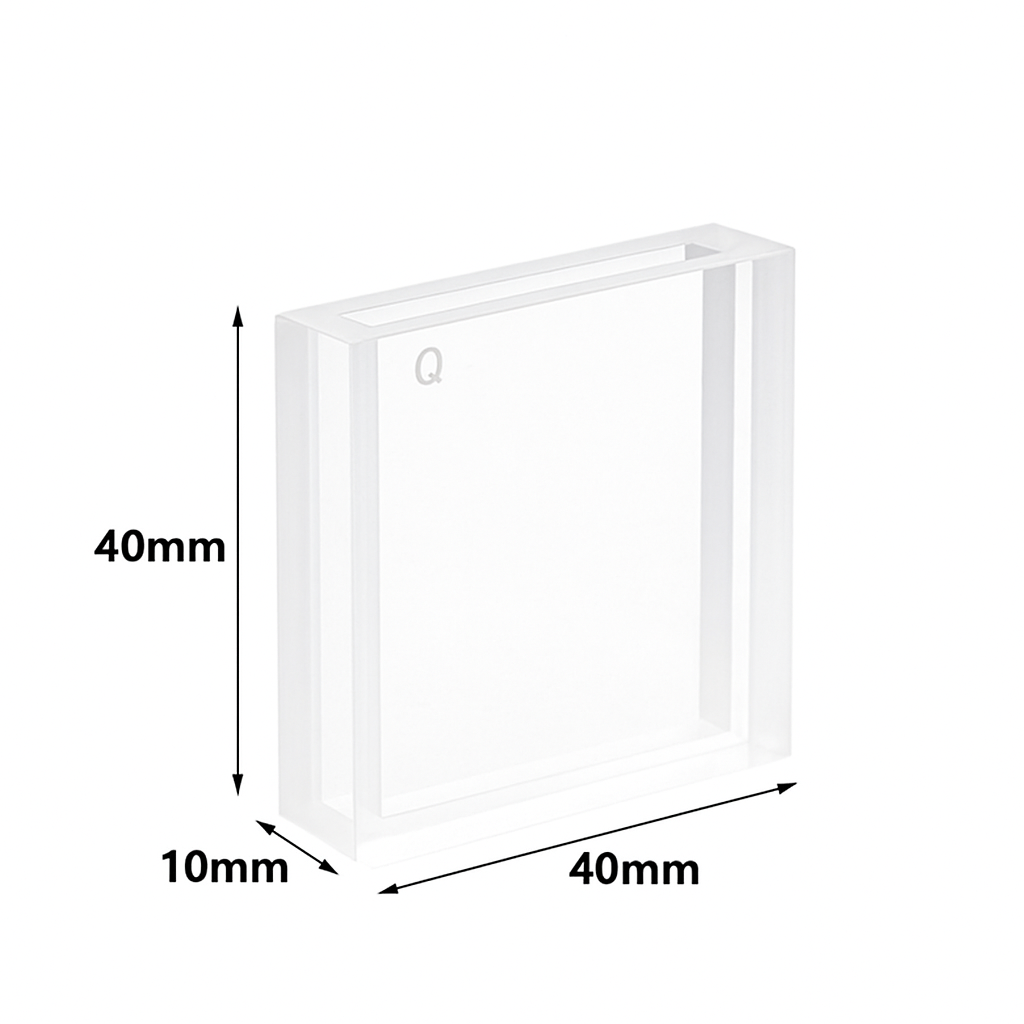
Standard Dimensions
To match most spectrophotometers, quartz cuvettes are built to standard sizes:
- Path length: 10 mm (global calibration standard for UV-Vis)
- Height: ~45 mm
- Volume: ~3.0–3.5 mL for a standard rectangular cell
For small or precious samples, semi-micro and micro-volume cuvettes are available. These reduce chamber volume but keep the standard 10 mm path length, so data remains comparable.
Optical Windows
The number of polished windows determines what experiments a cuvette can be used for:
- 2-window cuvettes: Front and back polished, sides frosted. Best for absorbance spectroscopy.
- 4-window cuvettes: All sides polished. Needed for fluorescence, since light enters from one side and is detected at 90°.
Why Quartz Matters
Quartz cuvettes provide clear advantages over glass or plastic:
- UV measurements (<300 nm): Essential for DNA (260 nm) and protein (280 nm) analysis
- Fluorescence spectroscopy: Very low background glow, so weak signals are easier to detect
- Chemical durability: Withstand solvents and reagents that damage plastic or glass
- Thermal stability: Safe up to 150–200 °C for fused cuvettes, ~1200 °C for molded fused silica cuvettes
For research and industrial labs, quartz cuvettes are not a luxury—they are the foundation for accurate and reproducible spectroscopy.
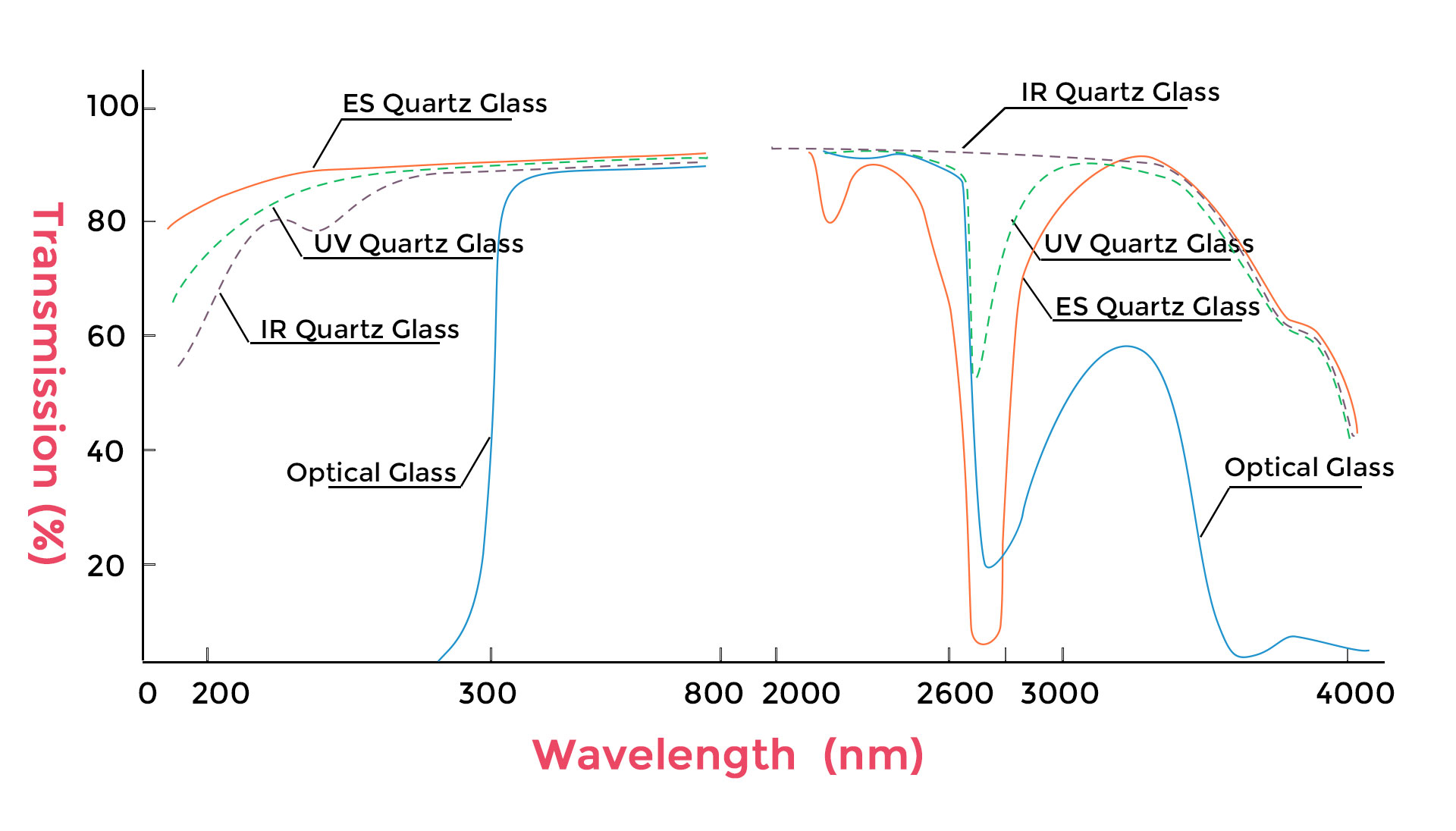
2. Quartz vs Glass vs Plastic Cuvettes
Glass and plastic cuvettes are still widely used in teaching laboratories and in situations where cost is a primary concern. However, each material has clear limitations. Understanding these trade-offs is essential for deciding when quartz is absolutely necessary and when a less advanced material may be acceptable.
UV Transmission
- Quartz: Provides excellent transmission from approximately 190 nm into the near-infrared (up to 2500 nm). This broad range is critical for UV-Vis spectroscopy and nucleic acid/protein analysis.
- Glass: Transparent in the visible region (350–2000 nm) but blocks most wavelengths below ~320 nm, making it unsuitable for DNA/RNA or protein quantification.
- Plastic: Usually transparent only in the visible range (400–800 nm) and blocks UV completely. Limited to colorimetric assays.
Autofluorescence
- Quartz: Very low autofluorescence, which makes it ideal for highly sensitive fluorescence spectroscopy.
- Glass: Produces moderate autofluorescence, which can interfere with weak signals and reduce sensitivity.
- Plastic: High autofluorescence, making it unsuitable for fluorescence work.
Cuvette Chemical Resistance
- Quartz: Resists most solvents, acids, and organic reagents, though it is incompatible with hydrofluoric acid.
- Glass: Offers moderate resistance but degrades when exposed to strong acids or bases for long-term storage.
- Plastic: Poor solvent resistance; readily attacked by acetone, ethanol, and DMSO, leading to deformation or dissolution.
Performance of Quartz Cuvettes (Molded) with Strong Acids and Bases
Strong Acids
Quartz (fused silica) has excellent chemical resistance and is generally stable against most strong acids, including hydrochloric acid (HCl), nitric acid (HNO₃), and sulfuric acid (H₂SO₄), at room temperature and elevated temperatures.
Exception: Hydrofluoric acid (HF) reacts with silica (SiO₂) and will corrode and dissolve quartz. Even low concentrations of HF should be avoided.
Strong Bases (e.g., NaOH, KOH)
Quartz exhibits higher alkali resistance than ordinary glass. Short-term exposure at room temperature is usually safe.
However, prolonged contact with concentrated strong bases—particularly under heating—can etch the quartz surface, reduce transparency, and compromise optical precision.
Conclusion
- Quartz cuvettes are suitable for handling most strong acids (except HF).
- They offer better resistance to strong bases than ordinary glass but are still vulnerable under long-term or high-temperature exposure.
- For applications involving HF or hot concentrated bases, PTFE (Teflon) cuvettes or other inert polymer materials are recommended.
Performance of Ordinary Glass Cuvettes (Molded) with Strong Acids and Bases
Strong Acids
Ordinary glass is stable against most strong acids (such as hydrochloric acid HCl, nitric acid HNO₃, and sulfuric acid H₂SO₄) at room temperature and can be used to contain them.
Exception: Hydrofluoric acid (HF) reacts with glass/quartz, corroding and dissolving it.
Strong Bases (e.g., NaOH, KOH)
Short-term contact at room temperature is generally not a problem.
However, strong bases—especially under heating—will gradually corrode glass, causing the surface to turn white and rough, which reduces transparency. Therefore, glass is unsuitable for long-term storage or high-temperature use with strong bases.
Conclusion
- Short-term storage of strong acids (except HF) is safe.
- Long-term storage or high-temperature use with strong bases will damage glass → not recommended.
- For handling strong bases or HF, it is better to use quartz cuvettes (heat-resistant but also not HF-resistant) or PTFE/polymer cuvettes.
| Chemical | PS | PMMA | UV Plastic | Glass Cuvette (Molded) | Quartz Cuvette (Molded) |
| Acetic acid, 100% | – | – | + | + | + |
| Acetone | – | – | + | + | + |
| Ammonia | + | + | + | + | + |
| Benzaldehyde | – | – | + | + | + |
| Butanone (methyl ethyl ketone, MEK) | – | – | + | + | + |
| Chloroform | – | – | – | + | + |
| Dioxane | – | – | + | + | + |
| DMF | – | – | + | + | + |
| DMSO | – | – | + | + | + |
| Ethyl Acetate | – | – | + | + | + |
| Hexane | – | + | – | + | + |
| Hydrochloric Acid, 36% | + | – | + | + | + |
| Hydrofluoric Acid, 10% | + | + | + | – | – |
| Isopropanol | + | + | + | + | + |
| Nitric Acid, 65% | – | – | + | + | + |
| Sodium Hydroxide | + | + | + | – | + |
Temperature Resistance
- Quartz: Stable up to 150–200 °C for fused cuvettes; molded quartz can withstand up to ~1200 °C.
- Glass: Limited to approximately 90 °C before deformation or cracking.
- Plastic: Restricted to ~60 °C; beyond this point the material warps or melts.
Durability and Cost
- Quartz: Highest upfront cost, but the longest lifespan when properly maintained. A single cuvette can last for years.
- Glass: Moderate price, with a usable life measured in months to years depending on handling and cleaning.
- Plastic: Lowest cost, designed for single-use or disposable applications.
Summary Table
| Feature | Quartz (Fused Silica) | Optical Glass | Plastic (PS/PMMA) |
|---|---|---|---|
| UV Transmission | Excellent (190–2500 nm) | Limited (>320 nm) | Not supported |
| Visible Transmission | Excellent | Excellent | Good |
| Autofluorescence | Low | Moderate | High |
| Chemical Resistance | High | Moderate | Low |
| Max Temperature | 150–1200 °C | ≤90 °C | ≤60 °C |
| Lifespan | Years (with care) | Months–Years | Disposable |
| Cost | Higher | Mid | Low |
| Best Use | UV-Vis, fluorescence, solvents | Visible-only assays | Teaching, colorimetric assays |
👉Conclusion
Plastic and glass cuvettes are serviceable for simple visible-light measurements, classroom demonstrations, or low-budget projects. For any experiment involving UV spectroscopy, fluorescence analysis, or solvent resistance, quartz is the only material that guarantees reliable, reproducible results.
3. Types of Quartz Cuvettes
Quartz cuvettes come in many designs. Each type is made for specific experiments, from routine UV-Vis absorbance to advanced fluorescence and kinetic studies. Choosing the right type improves accuracy, saves time, and keeps results reproducible.
Standard Rectangular Quartz Cuvettes (10 mm Path Length)
- Design: Rectangular body with two polished windows (front and back) and frosted sides for handling. Volume ~3–3.5 mL.
- Applications: UV-Vis absorbance, teaching labs, pharmaceutical QC, general research.
- Advantages: Fits most instruments, strong, easy to clean and reuse.
- Scientific note: 10 mm is the global standard path length. It balances sensitivity and convenience.
- Practical note: Needs ~3 mL sample. Not ideal for costly reagents or rare samples.
Case example: Teaching labs often use standard cuvettes because they resist heavy use and give consistent results.
Fluorescence Quartz Cuvettes (4-Window)
- Design: Four polished windows. Light enters one side and emission is detected at 90°. Optional PTFE or silicone caps reduce evaporation.
- Applications: Fluorescence, scattering, FRET studies.
- Advantages: Very low background glow, good for weak signals.
- Scientific note: Side windows are needed because fluorescence is measured at right angles. Quartz minimizes unwanted glow.
- Practical note: Handle carefully. Even small smudges on side windows affect emission data.
Case example: A protein–ligand study switched from glass to quartz. Signal-to-noise improved, letting them detect weak binding.
Semi-micro Quartz Cuvettes
- Design: Narrower chamber, ~0.35–1.75 mL volume, but path length remains 10 mm.
- Applications: Protein measurement with limited samples, pharma assays with costly reagents.
- Advantages: Saves sample while keeping standard path.
- Scientific note: Same optical geometry as standard cuvettes.
- Practical note: Needs careful pipetting to avoid bubbles.
Case example: A biotech lab used semi-micro cuvettes for antibody assays, reducing waste of expensive proteins.
Micro-volume Quartz Cuvettes
- Design: Very narrow channels, volume 10–700 µL.
- Applications: DNA/RNA measurement, enzyme studies with rare or costly samples.
- Advantages: Works with very small sample sizes.
- Scientific note: Narrow channels extend the path, making small samples measurable.
- Practical note: Fill slowly to avoid bubbles. Pre-rinse with buffer to reduce sample sticking.
Case example: A genomics team measured RNA from single-cell samples using micro-volume cuvettes.
Flow-through Quartz Cells
- Design: Inlet and outlet ports (M6-1 thread) for continuous flow. Some have jackets for temperature control.
- Applications: Kinetics, reaction monitoring, HPLC systems.
- Advantages: Allows real-time, automated measurements.
- Scientific note: Keeps sample moving so data can be collected without refilling.
- Practical note: Needs proper tubing and tight seals to avoid leaks.
Case example: A QC lab used flow-through cuvettes for drug dissolution testing, tracking release in real time.
Long-path Quartz Cuvettes
- Design: Extended chamber for 20–100 mm path length.
- Applications: Environmental testing, trace analyte detection at very low levels.
- Advantages: Longer path gives higher sensitivity for dilute samples.
- Scientific note: Beer–Lambert law: longer path = higher absorbance signal.
- Practical note: Fragile and needs special holders.
Case example: An agency used 50 mm cuvettes to detect nitrates in water at parts-per-billion levels.
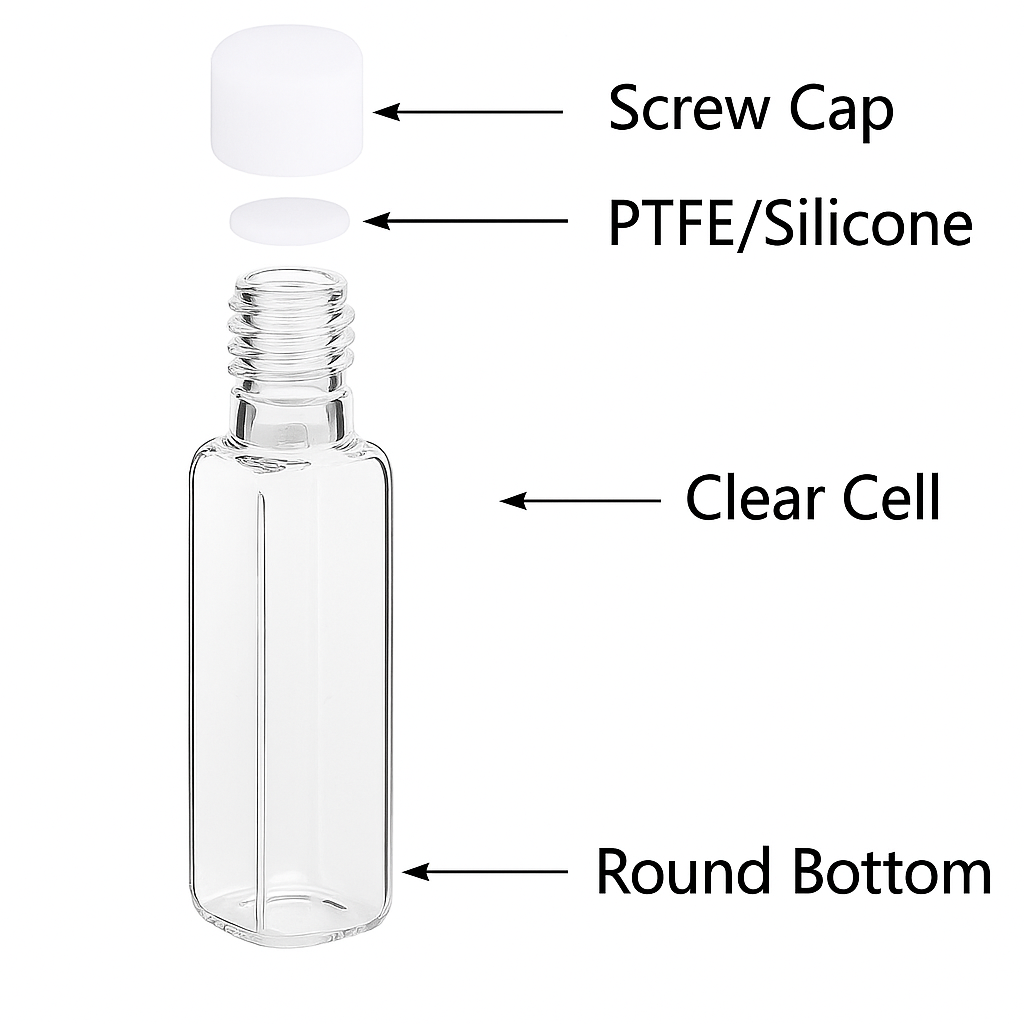
Sealed and Screw-cap Quartz Cuvettes
- Design: Standard quartz body with PTFE or silicone caps, sometimes with stoppers or septa.
- Applications: Volatile solvents (ethanol, chloroform) or oxygen-sensitive samples.
- Advantages: Prevents evaporation and contamination.
- Scientific note: Open-top cuvettes allow solvent loss and oxygen entry. Caps solve this issue.
- Practical note: PTFE resists most solvents; silicone is flexible but less resistant.
Case example: A biochemistry lab studying oxygen-sensitive enzymes used sealed cuvettes to prevent oxidation.
Custom Quartz Cuvettes
- Design: Made to order: dimensions, geometry, windows, coatings, ports.
- Applications: Specialized spectroscopy, OEM instruments, unique research needs.
- Advantages: Tailored for exact requirements.
- Scientific note: Standard designs cannot cover all experiments. Custom work ensures fit and accuracy.
- Practical note: Takes 3–6 weeks and needs technical drawings.
- Case example: An instrument maker ordered custom 4-window flow-through cuvettes with reinforced windows for fluorescence detection.
Summary
Quartz cuvettes range from standard 10 mm cells to advanced custom builds. They support everything from teaching labs to high-level industrial R&D. Picking the right type ensures accuracy, saves samples, and keeps experiments reproducible.
4. How to Select the Right Quartz Cuvette
Choosing the right cuvette means matching your experiment with the correct design. A wrong choice wastes time, samples, and money, and can reduce accuracy.
Key Decision Factors
Wavelength Range
- For UV work below 300 nm, quartz is required. DNA at 260 nm and proteins at 280 nm cannot be measured in glass or plastic.
- For visible-only tests (like food dye or classroom demos), glass or plastic may be enough.
Technique
- Absorbance assays → 2-window quartz cuvette
- Fluorescence spectroscopy → 4-window quartz cuvette (needed for side detection)
- Kinetics or continuous monitoring → Flow-through quartz cell
- Trace detection → Long-path quartz cuvette
Sample Volume
- Plenty of sample → Standard cuvette (~3.5 mL)
- Limited sample → Semi-micro cuvette (~1.2 mL)
- Very small or precious sample → Micro-volume cuvette (<1 mL)
Path Length
- Standard: 10 mm (default for most instruments)
- High concentration → Shorter path (1–5 mm) prevents oversaturation
- Very dilute sample → Longer path (20–100 mm) boosts sensitivity
Sealing & Environment
- Volatile or oxygen-sensitive samples → Sealed or screw-cap cuvettes prevent loss or oxidation
- Continuous flow → Threaded flow-through cuvettes connect to pumps or HPLC systems
Decision Table
| Application | Recommended Cuvette | Reason |
|---|---|---|
| DNA measurement at 260 nm | Micro-volume quartz cuvette | Saves rare samples |
| Protein absorbance at 280 nm | Semi-micro quartz cuvette | Uses less sample, keeps 10 mm path |
| Enzyme kinetics | Flow-through quartz cell | Real-time data, no refilling |
| Trace pollutant in water | Long-path quartz cuvette | Higher sensitivity |
| Fluorescence spectroscopy | 4-window quartz cuvette with cap | Reduces background, prevents evaporation |
| Teaching labs | Standard 10 mm quartz cuvette | Robust and universal |
Mistakes to Avoid
- Using plastic for UV: plastic blocks UV, so DNA/RNA results are invalid.
- Choosing the wrong path: short paths make dilute samples unreadable.
- Ignoring sealing: volatile solvents evaporate, skewing results.
- Overlooking instrument fit: long-path cuvettes may not fit standard holders.
Expert Tip
Always match sample concentration with path length.
- For high concentrations → use shorter paths to avoid oversaturation (OD > 2).
- For low concentrations → use longer paths to get measurable signals.
This follows the Beer–Lambert law (A = εcl), where absorbance is proportional to both concentration and path length. Correct path selection keeps results within your instrument’s linear range.
5. Technical Specifications & Tolerances
Quartz cuvettes are not simple blocks of quartz. They are precision optical tools. Even very small errors in size, polish, or alignment can affect your results. When comparing suppliers, it is important to understand which specifications matter most for accuracy and reliability.
Path Length Tolerance
The path length is the distance between the inner faces of the transparent windows. Standard cuvettes are designed for 10 mm, but tolerance is critical:
- High-precision cuvettes: ±0.01 mm
- Standard cuvettes: ±0.05 mm
Why it matters: According to the Beer–Lambert law (A = εcl), absorbance is proportional to path length. A small error of 0.05 mm can introduce significant mistakes in DNA, RNA, or protein measurements.
Window Flatness and Parallelism
Windows must be flat and parallel to ensure the light beam passes through correctly.
- Poor flatness → increases scattering, weakens the signal
- Poor parallelism → bends the light, adds baseline noise
Think of it like looking through a warped glass window: the view bends. The same happens inside a spectrophotometer cuvette.
Comparison of parallel vs. misaligned cuvette windows
| Feature | Parallel Windows (Standard Cuvette) | Misaligned Windows (Flawed Cuvette) |
|---|---|---|
| Light path | The light beam enters and exits the cuvette in a straight line, parallel to the path of the instrument. This is essential for accurate absorbance measurements governed by the Beer-Lambert law. | The light beam is refracted and displaced as it passes through the angled walls. This causes the light to exit at a different position than intended. |
| Measurement accuracy | Provides highly accurate and reproducible measurements. Quality-certified cuvettes have a pathlength that is certified to a high degree of precision. | Inaccuracy occurs because the light beam is not centered on the detector, leading to incorrect absorbance or fluorescence readings. The amount of light selected by downstream slits can also change. |
| Precision | Ensures high precision by minimizing the effect of refractions and optical aberrations. For highly sensitive techniques like FCS, a perfectly parallel alignment between the cuvette window and objective lens is critical for the best signal. | Leads to poor measurement precision. Rotating a cuvette with non-parallel windows can cause the signal intensity to change, which is a sign of a flawed cuvette. |
| Scattering | Minimizes light scattering, as the smooth, parallel surfaces allow for a direct path of light. This is especially important for turbidity measurements. | Significantly increases light scattering. The angled surfaces cause light to scatter rather than pass straight through, which can cause interference and poor signal quality. |
| Impact on instrument | Assumes and relies upon the proper alignment of the cuvette to the instrument’s optical components. Any deviation from this optimal setup can cause problems. | Can compromise the sensitivity of the entire optical system. For example, in FCS, an angular misalignment as small as 0.40 degrees has been shown to decrease sensitivity significantly. |
| Application | Suitable for most quantitative optical measurements, including UV-Vis spectrophotometry, fluorescence spectroscopy, and fluorescence correlation spectroscopy. | Generally unusable for high-precision optical measurements. A cuvette with misaligned windows is a defective product that should be replaced. |
Optical Polish
The polish of the window surface controls how much stray light is produced.
- High-quality cuvettes: polished to λ/4 or better → smooth and clear
- Low-grade cuvettes: fire-polished → may look fine but cause scatter and noisy baselines
This is especially important in fluorescence studies, where poor polish can hide weak signals.
Temperature Limits
Quartz itself tolerates ~1200 °C, but the cuvette design limits real use:
- Molded quartz cuvettes: up to ~1200 °C
- Fused quartz cuvettes: ~150–200 °C
- Glued cuvettes: only ~80–120 °C (adhesives fail earlier)
Going beyond these limits risks cracks or window detachment, which can also damage the instrument holder.
Advantages of Quartz cuvettes on Thermal Stress
- High thermal stability: Quartz cuvettes are the most resilient to temperature changes, including rapid thermal shock.
- Withstands thermal shock: High-quality quartz, like Suprasil, can endure rapid temperature changes, making it ideal for cryogenic applications or experiments that involve heating.
- Structural integrity: Unlike plastic, quartz maintains its shape under high heat, which ensures consistent path length and measurement accuracy during temperature-dependent experiments.
- Chemical resistance: While very resistant, quartz can be degraded by strong bases or hydrofluoric acid (HF) under long-term or high-temperature exposure.
Chemical Compatibility
Quartz is resistant to most acids, bases, and solvents. But there are exceptions:
- Hydrofluoric acid (HF): This is the only common acid that dissolves quartz (silicon dioxide). It can etch and corrode quartz even at low concentrations and room temperature. This reaction is often utilized for controlled etching in the semiconductor industry.
- Hot concentrated phosphoric acid (H₃PO₄): While phosphoric acid has little effect on quartz at room temperature, it will attack and damage the surface at temperatures above 150 °C.
- Strong alkalis (e.g., sodium hydroxide (NaOH) and potassium hydroxide (KOH)): At room temperature, quartz has good resistance to most bases. However, long-term exposure to strong alkali solutions, especially at high temperatures, will slowly etch the quartz surface.
Risks with bonded quartz cuvettes
In addition to the inherent chemical vulnerabilities of quartz itself, bonded cuvettes present another risk. These cuvettes use a bonding material, or glue, to assemble the individual quartz plates. These bonds can be compromised by a range of solvents and corrosive solutions that the quartz itself would resist.
- Compromised bonds: Solvents like benzene, toluene, and ethanol can attack the adhesive, causing the cuvette to leak.
- Alternative for resistance: If your application requires greater chemical resistance, choose fused or molded cuvettes, which are assembled using heat and pressure rather than adhesive.
Compatibility of fused vs. bonded quartz cuvettes
| Solution type | Fused or molded quartz cuvettes | Bonded quartz cuvettes (adhesive-dependent) |
|---|---|---|
| Common acids e.g., HCl, HNO₃, H₂SO₄ | Compatible. The fused quartz is stable against most strong acids at room and elevated temperatures. | Compatible with the quartz, but extended exposure to high concentrations can weaken or dissolve the adhesive. |
| Hydrofluoric acid (HF) | Incompatible. Attacks and dissolves the quartz material. | Incompatible. The quartz material is not resistant to HF. |
| Hot concentrated phosphoric acid | Incompatible at temperatures over 150 °C. The acid will damage the quartz surface. | Incompatible for the same reason, and hot conditions will also degrade the adhesive. |
| Strong alkalis e.g., NaOH, KOH | Generally compatible for short-term use. Prolonged exposure to concentrated bases, especially at high temperatures, will etch the quartz. | Generally compatible for short-term use. The adhesive is likely to fail under long-term or high-temperature exposure. |
| Organic solvents e.g., acetone, toluene, chloroform | Compatible. The fused silica is resistant to a wide range of common organic solvents. | Incompatible with many adhesives. Solvents can degrade the bonds, causing the cuvette to leak. |
| Aqua regia | Compatible. High-grade fused cuvettes are resistant to aqua regia. | Incompatible. The adhesive will be dissolved by this highly corrosive mixture. |
Always confirm chemical compatibility before long-term use.
Seal and Cap Materials
Seal choice affects both chemical resistance and long-term stability:
- PTFE: inert and solvent-resistant, best for strong chemicals
- Silicone: flexible, easy to use, but less resistant
- Screw caps: good for volatile or oxygen-sensitive samples
- Custom seals: such as O-rings, available for high-pressure or special uses
Specification Table
| Parameter | Typical Value | Why It Matters |
|---|---|---|
| Path length tolerance | ±0.01–0.05 mm | Direct impact on absorbance accuracy |
| Window parallelism | ≤5 arc minutes | Reduces baseline noise |
| Optical polish | λ/4 or better | Prevents scatter |
| Temperature limit | 150–1200 °C (molded) | Adhesives or seals set the limit |
| Chemical resistance | Excellent, except HF and hot alkalis | Ensures long life |
| Seal materials | PTFE, silicone, epoxy | Affects solvent compatibility |
Case Example: Poor Tolerances in Practice
A research lab purchased low-cost quartz cuvettes online for protein assays at 280 nm. At first, they looked fine. But results showed baseline drift and inconsistent absorbance. Later checks revealed path length variations of 0.1 mm. This small error caused 5–10% mistakes in protein concentration.
After switching to high-precision cuvettes (±0.01 mm), results became stable and reproducible.
👉 Lesson: Tolerances matter as much as material. Always check manufacturer specifications before purchase.
6. Applications & Case Studies
Quartz cuvettes are used in many fields of science. Their key strengths—deep UV transparency, chemical resistance, heat stability, and very low background glow—make them the first choice whenever accuracy matters. Below are the main areas where quartz cuvettes are essential, with real-world examples.
1. Molecular Biology
DNA and RNA Measurement
Nucleic acids are measured at 260 nm, a wavelength blocked by glass or plastic. Only quartz passes this light without distortion. Micro-volume quartz cuvettes make it possible to measure with just 1–5 µg of nucleic acids, saving rare material.
Protein Measurement
Proteins absorb at 280 nm due to tryptophan and tyrosine residues. Semi-micro quartz cuvettes save sample volume while keeping the 10 mm path length for accurate results.
Case Example
A genomics lab working with rare patient samples used 350 µL micro-volume quartz cuvettes. This let them perform DNA and RNA tests without using up all of their limited material.
Best practices for accurate results
- Be cautious with small volumes: Work carefully when pipetting very small volumes to avoid errors.
- Use the right cuvette: The use of a quartz cuvette is critical for accurate measurements in the UV range (220–350 nm). Plastic cuvettes absorb UV light and can distort results.
- Avoid contamination: Clean the cuvette and pipette tips between samples to prevent cross-contamination.
2. Fluorescence Spectroscopy
Quartz is the preferred material for fluorescence spectroscopy due to its exceptionally low background luminescence, which minimizes noise in sensitive measurements. Cuvettes with four optical windows enable excitation and emission detection at a 90° angle, effectively eliminating interference from direct light paths.
Typical applications include:
- Enzyme kinetics studies
- Förster Resonance Energy Transfer (FRET) assays
- Small-molecule interaction and binding experiments
- Environmental monitoring using fluorescent dyes
Case Example
A biochemistry research team investigating protein–ligand interactions discovered that standard glass cuvettes introduced unwanted background signals. By switching to four-window quartz cuvettes, they eliminated this interference and successfully detected weak binding events that would otherwise have been obscured.
3. Pharmaceutical Research & Development
Quartz cuvettes play a pivotal role in pharmaceutical laboratories, where both accuracy and reproducibility directly impact drug safety and regulatory compliance. From early-stage drug discovery to large-scale production, their unique optical properties and chemical stability ensure reliable results that other materials cannot provide.
Key Applications
- Dissolution Testing
In formulation development and production, flow-through cuvettes combined with pumps enable continuous monitoring of how tablets and capsules release active pharmaceutical ingredients (APIs). Real-time absorbance data provides critical insights into bioavailability and helps ensure that products meet pharmacopeia standards (e.g., USP, EP, JP). - Drug Stability Studies
Ultraviolet (UV) absorbance measurements are widely used to monitor chemical degradation pathways and shelf-life under stress conditions such as heat, light, and humidity. Quartz cuvettes, with deep-UV transparency down to ~190 nm, allow detection of subtle changes in molecular structure that glass or plastic would miss. - Automated Quality Control Assays
Modern pharmaceutical plants rely on high-throughput automated systems for batch testing. Quartz cuvettes are integrated into robotic platforms, spectrophotometers, and microplate readers to deliver precise, reproducible data across thousands of samples—minimizing variability and human error.
Case Example
A pharmaceutical quality control (QC) laboratory upgraded its dissolution testing workflow by incorporating Qvarz flow-through quartz cuvettes into automated dissolution systems. This innovation provided several measurable benefits:
- Real-time drug release tracking with continuous absorbance monitoring
- Improved operational efficiency, reducing analysis time per batch
- Minimized operator error, as manual sampling and transfers were eliminated
- Regulatory compliance assurance, with consistent data output that aligned with FDA and EMA validation requirements
By adopting quartz cuvettes in both R&D and production quality checks, the lab not only accelerated decision-making but also enhanced overall product reliability—critical for patient safety and faster time-to-market.
4. Environmental Analysis
Detecting pollutants at trace levels requires extremely high sensitivity. Long-path quartz cuvettes (20–100 mm) are specifically designed to increase the optical path length, thereby amplifying weak absorbance signals that would otherwise fall below detection limits. This makes them indispensable in environmental monitoring, where accuracy at parts-per-billion (ppb) levels can determine compliance with health and safety standards.
Typical Analyses
- Nitrates and Nitrites – linked to agricultural runoff and groundwater contamination
- Phosphates – indicators of eutrophication in lakes and rivers
- Heavy Metals (e.g., lead, cadmium, arsenic) – toxic even at trace concentrations
- Organic Pollutants – including pesticides, industrial solvents, and hydrocarbons
Case Example
An environmental protection agency implemented 50 mm path length quartz cuvettes in routine water quality assessments. By extending the optical path length, their spectrophotometers achieved the sensitivity required to detect nitrates in drinking water at parts-per-billion concentrations. This capability ensured compliance with World Health Organization (WHO) safety guidelines and enabled early detection of agricultural runoff before it posed public health risks.
5. Material Science & Industrial Research
Quartz’s exceptional stability under high heat, aggressive chemicals, and intense light exposure makes it indispensable in advanced material science and industrial research. Its optical clarity, durability, and resistance to deformation under stress allow experiments that demand both precision and robustness.
Key Applications
- Thin-Film and Coating Analysis
Quartz cuvettes provide a chemically inert and optically transparent container for studying the optical properties of thin films, coatings, and nanomaterials—especially where UV–visible or IR spectroscopy is required. - High-Energy Laser Experiments
Due to its high damage threshold and low autofluorescence, quartz withstands exposure to pulsed and continuous-wave lasers. This makes it essential for experiments in laser-material interactions, optical device testing, and nonlinear optics. - Light Scattering & Refractive Index Studies
The uniform optical quality of quartz cuvettes enables accurate measurements of scattering, birefringence, and refractive index, all critical for polymer science, colloid stability testing, and advanced photonic research.
Case Example
A university materials research group designed a custom high-pressure quartz cuvette with reinforced windows to study polymer samples under compression. Thanks to quartz’s unique combination of mechanical strength and optical clarity, the team was able to observe structural changes in real time using laser scattering methods—an experiment that would have been impossible with glass or plastic cells.
6. Clinical & Diagnostic Applications
In clinical laboratories, precision and reliability are non-negotiable, as even small errors can directly affect patient outcomes. Quartz cuvettes provide the optical stability and chemical resistance required for assays that must consistently deliver accurate data across thousands of tests.
Key Applications
- Blood and Serum Assays
Quartz’s transparency in both UV and visible ranges allows accurate detection of analytes such as bilirubin, hemoglobin, and lipids, which are critical markers in routine blood chemistry panels. - Enzyme Activity Testing
Enzymes often have strong absorbance or fluorescence signals in the UV range. Quartz cuvettes ensure reproducibility when monitoring enzyme kinetics, inhibitor effects, or metabolic pathways. - Diagnostic Development
From infectious disease assays to next-generation biomarker discovery, stable quartz optics enable the development and validation of diagnostic tools where signal clarity determines test sensitivity and specificity.
Why Quartz Matters
Unlike plastic or glass, quartz transmits deep-UV wavelengths and resists degradation under repeated sterilization or chemical exposure. This ensures long-term optical integrity, reducing background noise and preventing false positives or negatives.
Case Example
A hospital laboratory integrating new UV-based diagnostic assays found that glass cuvettes introduced baseline drift, leading to inconsistent results. By switching to quartz cuvettes, the lab eliminated this variability, ensuring reproducible readings across multiple instruments—providing physicians with the confidence needed for accurate patient care decisions.
7. Summary of Applications
| Field | Application | Recommended Cuvette |
|---|---|---|
| Molecular Biology | DNA/RNA and protein measurement | Micro or semi-micro quartz cuvette |
| Biochemistry | Enzyme kinetics, FRET | 4-window quartz cuvette |
| Pharma R&D | Drug stability, dissolution testing | Flow-through quartz cell |
| Environmental | Pollutant and water analysis | Long-path quartz cuvette |
| Materials Science | Thin films, lasers, high pressure | Custom quartz cuvette |
| Clinical Testing | Blood/serum assays, enzyme tests | Standard 10 mm quartz cuvette |
7. Quartz Cuvettes and Cells – Exploring the Types
8. Extended Frequently Asked Questions (FAQ)
Over the years, researchers have asked us hundreds of questions about quartz cuvettes. Below is a curated list of the most common, along with detailed answers.
Q1. Why are quartz cuvettes better than glass?
Quartz transmits UV light down to ~190 nm, while glass cuts off at ~320 nm. This makes quartz essential for DNA/RNA (260 nm) and protein (280 nm) absorbance. Quartz also has lower autofluorescence and better chemical resistance.
Q2. What path length should I use?
- 10 mm is the universal standard (most instruments are calibrated for it).
- Use shorter paths (1–5 mm) for concentrated samples to avoid absorbance saturation.
- Use longer paths (20–100 mm) for dilute or trace analytes.
Q3. Can quartz cuvettes be used with organic solvents?
Yes. Molded / fused quartz is resistant to most organics (ethanol, acetone, DMSO, methanol, chloroform), while glued quartz cuvette is for aqueous samples only. PTFE seals are preferred for aggressive solvents.
Q4. Can quartz cuvettes be autoclaved?
Generally, no. While molded / fused quartz withstands heat, glued cuvettes contain adhesives or seals that degrade at autoclave temperatures (~121 °C under pressure). Instead, clean with solvent rinses.
Q5. How long does a quartz cuvette last?
With proper care, quartz cuvettes can last years, even decades. Most failures are due to scratches, chemical attack, or mishandling—not material fatigue.
Q6. Can quartz cuvettes be repaired or repolished?
Minor scratches can sometimes be polished out by specialized optics shops. However, for most labs, replacement is more cost-effective. Deep scratches or chipped edges usually mean the cuvette is no longer reliable.
Q7. Do quartz cuvettes need calibration?
Not in the sense of instruments. However, path length verification is recommended. High-end cuvettes are supplied with certificates showing measured path length. For critical assays, choose cuvettes with ±0.01 mm tolerance.
Q8. How are path lengths verified?
Manufacturers measure cuvettes using interferometry or precision gauges. In labs, approximate verification can be done using standards (e.g., potassium dichromate solutions at known concentrations).
Q9. How do I avoid bubbles inside cuvettes?
- Fill slowly down one side of the wall.
- Degas samples if possible.
- Use micro-pipette tips to introduce liquid smoothly.
- Gently tap the cuvette to release trapped bubbles.
Q10. Can quartz cuvettes be used in IR spectroscopy?
Yes. Quartz transmits into the near-IR up to ~2500 nm. For mid-IR (>3000 nm), materials like CaF₂ or ZnSe are used instead. Quartz is ideal for UV-Vis-NIR applications.
Q11. What’s the difference between synthetic and natural quartz?
- Natural quartz: mined, may contain impurities that interfere with optical quality.
- Synthetic (fused silica): manufactured for purity and reproducibility; always used for optical-grade cuvettes.
Q12. What sealing options are available?
- Open-top cuvettes (no cap), PTFE lids cuvettes
- Stopper lid cuvettes
- Screw-cap cuvettes (PTFE, silicone seals)
- Septa for injection sampling
- Flow-through ports (M6-1 threads, custom fittings)
Q13. What’s the maximum temperature for quartz cuvettes?
- Standard fused cuvettes: 150–200 °C
- With special seals: higher, but still limited by adhesive materials
- Molded quartz cuvettes: ~1200 °C
Q14. How should quartz cuvettes be stored?
- Store upright in a padded box
- Keep capped to prevent dust
- Label by application (e.g., “DNA only”)
- Avoid high humidity environments
Q15. Can quartz cuvettes be disposable?
Yes, disposable quartz cuvettes exist for high-throughput labs. They are thinner and cheaper, but less durable. Most labs prefer reusables due to cost efficiency.
Q16. How are quartz cuvettes shipped safely?
Cuvettes are typically shipped in foam-lined boxes, individually wrapped to prevent chipping. For international shipping, double boxing with cushioning is standard.
Q17. Are custom shapes possible?
Yes. Custom options include:
- Non-standard path lengths (2 mm, 20 mm, 100 mm)
- Special geometries (triangular, cylindrical)
- Extra-thick windows for high-pressure work
- Temperature-jacketed cells
Q18. How do quartz cuvettes compare in price?
- Standard 10 mm: mid-range cost
- Fluorescence or micro-volume: higher due to precision machining
- Flow-through/long-path: higher, especially with custom fittings
- Custom builds: price varies with complexity
Q19. What are the lead times for custom fabrication?
Typically 3–6 weeks, depending on complexity and fabrication methods. Urgent orders may be expedited.
Q20. Do all quartz cuvettes fit all spectrophotometers?
Most instruments use 10 mm holders, but always confirm height and outer dimensions. Long-path cuvettes or flow cells may require special adapters.
Summary:
Quartz cuvettes are versatile but specialized. Understanding their limits—temperature, solvent compatibility, cleaning methods—ensures maximum lifespan and data quality.
9. Related Resources
When learning about quartz cuvettes, it’s often useful to connect the dots with related topics. Below are resources and concepts that expand your understanding:
9.1 Fluorescence Quartz Cuvette Guide
- Covers why 4-window designs are required for 90° detection
- Explains low-autofluorescence requirements in protein and dye assays
- Helps researchers avoid background interference issues
9.2 Flow-through Quartz Cells
- Introduces continuous monitoring of reactions
- Integration with HPLC and peristaltic pumps
- Case examples: enzyme kinetics, dissolution testing in pharma
9.3 Custom Quartz Fabrication Services
- Overview of bespoke options: unusual path lengths, ports, coatings
- Tailored for OEM partners and advanced R&D labs
- Practical for labs that require more than off-the-shelf solutions
9.4 Spectroscopy Fundamentals
- Refreshers on Beer–Lambert law and absorbance
- Impact of path length and concentration on signal quality
- Why cuvette material choice is as important as instrument calibration
9.5 Care & Maintenance Guidelines
- Advanced tips for cleaning, including ultrasonic baths
- Troubleshooting tables for common problems
- Guidance for extending cuvette lifespan
👉 By linking these resources together, you create a knowledge ecosystem around quartz cuvettes, strengthening both your scientific workflow and SEO topical authority.
10. Conclusion
Quartz cuvettes are more than just containers; they are precision optical tools that can make or break your experimental results. By choosing the right cuvette—whether standard, fluorescence, micro-volume, flow-through, or long-path—you ensure accuracy, sensitivity, and reproducibility.
- What quartz cuvettes are and why they matter
- How quartz compares to glass and plastic alternatives
- The full range of cuvette types and their specialized uses
- Key specifications and tolerances that influence accuracy
- Real-world applications across biology, chemistry, pharma, and industry
- FAQs addressing the most common researcher questions
The bottom line: If you are working in UV-Vis or fluorescence spectroscopy, quartz cuvettes are essential. They combine durability, precision, and optical clarity, ensuring that your data reflects science—not artifacts.
👉 Ready to take your experiments to the next level? Request a Quote for Quartz Cuvettes Today
Disclaimer
The information provided in this document is for general informational and educational purposes only. While every effort has been made to ensure accuracy, Qvarz does not warrant or guarantee the completeness, reliability, or suitability of the information for any specific application.
Use of quartz cuvettes and related laboratory products should always follow applicable safety guidelines, regulatory standards, and the instructions provided by the instrument manufacturer. Qvarz shall not be held liable for any direct, indirect, or consequential damages arising from the use or misuse of the products or information described herein.
All product specifications are subject to change without prior notice. Mention of third-party applications or case examples is illustrative only and does not constitute an endorsement.






































Recent Comments